|
ZHENGZHOU HEPO INTERNATIONAL
|
Fetal Doppler Ultrasound Fetal Heart Monitor
| Price: | 1160.0 USD |
| Payment Terms: | T/T |
| Place of Origin: | Henan, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
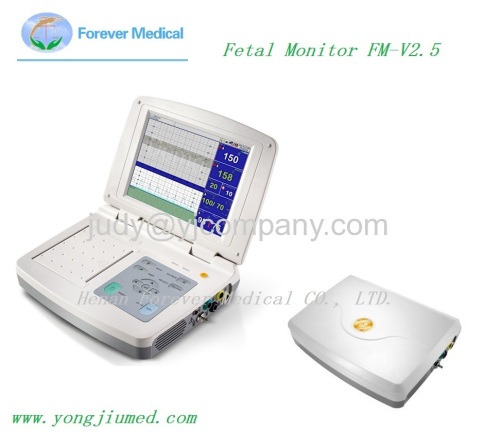
The 10.4 inches high-tech fetal monitor offers a reliable, high-performance solution for fetal monitoring needs in doctors' practices
10.4 Inches Fetal Monitor (FM-V2.5)
Technical Data Sheet - Release V2.5
The 10.4 inches high-tech fetal monitor offers a reliable, high-performance solution for fetal monitoring needs in doctors' practices, clinics and hospitals, from pre-natal check-ups to antepartum monitoring of high-risk pregnancies.
An optional USB Mouse/Keyboard control eases navigation and data entry.
An optional USB port offers patient data/NST report backup.
An optional Non-Stress Test (NST) report software eases documentation in antepartum monitoring.
Features
Core
Newly advanced A9 main board with Linux OS, calculating speed is 4 times faster than traditional products
Support storage of 25000 groups of medical record and 8640 hours trend review
Body
10.4 inch Folded high-brightness TFT LED
Optional touch screen
Sensor
12-Crystal ultrasound transducer achieves a wider focal region for more uniform coverage at greater depths than conventional 9-crystal. 12-crystal ultrasound design can also get good result even when the patient varies her position and shape
Optional waterproof FHR transducer for underwater birth
Event Marker for easy documentation of events and kick counts. Auto-fetal-movement detector is also available.
Use of signal strength indicator to position the ultrasound transducers at the optimum location on the patient
Printer
Built-in high-speed thermal printer, printing width can be set to 112mm (Brand: SEIKO, Japan)
Support fast printing of stored trend
Central System
Optional Build-in wireless network module, supporting wired or wireless connection to the central monitoring station.
Optional Support HL7 (Health Level Seven)
Alarm
Three-level acousto-optic alarm
FHR signal strength prompt
Cross-Channel Verification (CCV) provides visual and audible indication when it detects synchronous fetal or maternal heart rate signals, indicating that you may be monitoring a duplicate signal)
Sensor-off alarm
Paper out alarm
Support alarm review
Linux OS
Support operation with mouse and computer keyboard (option)
Multi-display mode to meet different clinical requirement, including Fetal-parameter interface, Maternal-parameter interface, Fetal/Maternal-parameter interface, as well as big font, alarm review, ECG review, trend table, trend graph and OxyCRG display.
NIBP self-test mode: including overpressure test, static pressure test and air leakage test.
On-screen scrolling for viewing stored fetal traces
Generate ID automatically when register a new patient
Support medical history search by patient ID, name and mobile number
NST Timer
Fetal Movement Profile (FMP) detects and automatically records gross fetal body movements
Heart rate offset mode allowing you to offset FHR2 ± 5~30BPM.
Support multi-language display
Advanced Fischer NST Report (Fetal Health Report)
Support online software upgrading by net/USB
Optional patient data &NST Report backup by USB in PDF format
Interface
Transducer socket
US1 (FHR1)
US2 (FHR 2)
TOCO
FM (Manual Fetal movement)
SPO2 (Maternal SpO2)
NIBP (Maternal NIBP)
LAN / Central monitor system interface
The LAN port is for connecting the monitor to a central monitor system network.
Input device interface
This optional interface provides an USB ports to enable the monitor to be connected to off-the-shelf input devices as well as support patient data & NST Report backup (option):
Mouse: any specified trackball or USB mouse may be used for navigation and data entry.
Computer keyboard: an USB computer keyboard can be used for data entry instead of the on-screen pop-up keyboard.
[Remark: user has to restart the machine after plug and unplug the mouse / computer keyboard.]
Touch screen (optional)
[Remark: Touch screen can not be used simultaneously with mouse / computer keyboard.]
Service Features
The Support Tool helps technical personnel to
- carry out configuration, upgrades and troubleshooting on an individual monitor.
back up the monitor settings.
The Service Mode is password-protected and ensures that only trained staff can access service tests and tasks. It includes Printer Setting, Skin Type, AFM Setting, Brightness Setting, Clear Data, NIBP Calibration, and Touch Screen Calibration.
The Support Tool uses the USB interface of the fetal monitor for software upgrading.
Related Product
The monitor is compatible with Acoustic Fetal Stimulator, which use low frequency vibro-acoustic stimuli (VAS) to "wake" the fetus, increasing the efficiency of antepartum testing and improving retest consistency.
The fetal monitors can be equipped with NST (Non Stress Test) Report function (option) based on Fischer evaluation method to give a score of fetal wellbeing after 20 minutes continuous monitoring, thus helping the doctors make judgment about fetal health condition.
Base line, LTV Amplitude, Period, Acceleration, and Deceleration are 5 indexes of analyzing FHR wave, the software allows interpretation of fetal heart Rate and TOCO traces, and generates a printed report automatically for a reassuring NST.
Performance Specifications
Dimension and Weight
Dimension: 320mm*260mm*80mm
Weight: 2.8kg(excluding accessories)
Power Supply
Adapter: Output 9VDC, 5A
Voltage: AC100~240V, 50/60HZ
Display
10.4" color TFT LED (0-135° rotation)
Resolution: 800*600 pixels
Battery (Built-in)
Type: Rechargeable lithium battery
Charge Cycle: ≥500 times
Working time: 2 hours (optional larger battery for 4-5 hours)
Recorder (Built-in)
Method: Thermal printer
Paper: Z-fold, thermosensitive
Paper width: 112 mm (4.41 in)
Printing speed: 1/2/3cm/min
Trace: Max. 4 tracks
Recording way: Real-time Recording, Review Printing
Alarm
Level: Low, medium and high
Indication: Auditory, Visual, Delay of FHR Alarm
Input Device
Touch screen (option)
Keypad input (Standard)
Mouse/ Keyboard input (option)
USB port for patient data/NST report backup (option)
System Output & Extensible Interface
Ethernet Network: 1 standard RJ45 socket
USB Port: 1
Safety
IEC60601-1 Approved, CE marking according to MDD93/42/EEC
With reference to RoHS Directive 2011/65/EU recasting
Trend & Reviewing
Cardiotocography (CTG):
Waveform Review: 8640 hours
Vital Signs:
Trend: 2160 hours
Waveform Review: 2 hours
NIBP Review: 2000 groups
Alarm Review: 2000 groups
Ultrasound (FHR)
Transducer type: 12-Crystal ultrasound transducer
Measure method: Wide beam pulse doppler
FHR Measurement range:
30 to 240 bpm (US Standard)
50 to 210 bpm (Europe Standard)
Accuracy: ±1 bpm
Intensity:<3 mW/cm2
Ultrasound frequency: 1MHz±3%
Resolution: 1 bpm
TOCO
Measurement method: Strain gauge sensor element
Measure range: 0 ~ 100 units
Resolution: 1 unit
Nonlinear deviation : ≤±8%
Baseline setting: 0,5,10,15, 20 can be selected
Auto offset correction: 3 second after connecting the transducer
Auto zero adjust: TOCO value is set to zero negative following a negative Measure value for 5 seconds
Fetal Movement Recording
Automatic fetal movement (AFM): pulsed doppler ultrasound
Automatic fetal movement autograph
Manual fetal movement marker (MFM)
Maternal SpO2 (Digital Technic)
Measurement Range: 0 ~ 100 %
Resolution: 1 %
Accuracy: ±2% (70% ~ 100%)
±3 % (35% ~ 69%)
Unspecified (0 ~ 34%)
Pulse Rate
Range: 25~250bpm
Accuracy: ±1% or ±1 bpm, whichever is greater
Resolution: 1bpm
Maternal NIBP (MNIBP)
Method: Oscillometric
Measure mode Manual, Auto, STAT
Measure Interval in AUTO Mode 1~480 min
STAT mode cycle time: Keep 5 minutes, at 5 seconds interval
Measure and Alarm Range:
Systolic: 30~270 mmHg
Diastolic: 10~220 mmHg
Mean: 20~235 mmHg
Static pressure accuracy ±3mmHg
Resolution: 1mmHg
Accuracy: Maximum Mean error ±5mmHg
Maximum Standard deviation 8mmHg
Over pressure Protection: Dual protection via software & hardware
NST Report/FHR Interpretation (Option)
Based on Fischer Evaluation Method (monitoring time≥20 Min)
Average Baseline FHR / Number of Acceleration / Number of Deceleration / Number of Small Acceleration / Amplitude Variation / Number of PV
Automatic Score Result: Sore: 8-10 (Excellent)
Sore: 5-7 (Suspicious)
Score:≤4 ( Fetus Hypoxia)
* Specifications subject to change without prior notice
Model Configuration
| Model | Fetal Parameter | Maternal Parameter | Printer Paper Width | Battery | ||||||||||||
| Single FHR | Dual FHR | TOCO | AFM & MFM | Signal Overlap Alarm | NIBP, SpO2, PR | ECG, TEMP, RESP | 112mm A type | 112mm B type | Lithium battery | |||||||
| 10.4 Inches Fetal Monitor (FM-V2.5) | Config. A | N/A | N/A | N/A | N/A | Opt | ||||||||||
| Config. B | N/A | N/A | Opt | |||||||||||||
| Config. C | N/A | N/A | Opt | |||||||||||||
| Config. D | N/A | Opt | ||||||||||||||
| Option: | 1) Touch Screen | 2) Fetal Stimulator | 3) Wall-mount | 4) Trolley | 5) Central Monitoring Station | |||||||||||
| 6) Waterproof FHR Probe & TOCO Probe | 7) Keyboard & Mouse control function | 8) Advanced Fischer NST Report | 9) USB port with patient data/NST report backup function | |||||||||||||
| Remark: The touch screen and mouse/ computer keyboard is not compatible by now. | ||||||||||||||||
| Table legend: : -Standard , Opt - Option, N/A - not applicable | ||||||||||||||||
Gallery for Optional Accessories:
| FHR Transducer | TOCO Transducer | Event Marker | Fetal Acoustic Stimulator | Ultrasound Gel (250g) | Reusable Transducer Belt |
| NIBP Cuff and NIBP Tube | Wall-mount with basket | Trolley with Basket | Central Monitoring Station | 112(A) mm Thermal Printer Paper (112mm×100mm×150p, European Standard, 30-210 bpm) | 112(B) mm Thermal Printer Paper (112mm×100mm×150p, US Standard, 30-240 bpm) |
Standard Packing List:
| Standard Packing List | Config. A | Config. B | Config. C | Config. D | ||
| No. | Parts No. | Description | QTY | QTY | QTY | QTY |
| 1 | 8310400004 | FHR Transducer | 1 | 2 | 1 | 2 |
| 2 | 8310400003 | TOCO Transducer | 1 | 1 | 1 | 1 |
| 3 | 8310400012 | Event Marker | 1 | 1 | 1 | 1 |
| 4 | 8189007066 | Belt | 2 | 3 | 2 | 3 |
| 5 | 8111810003 | Recording Paper (112mmx100mmx150p, European Standard) | 2 | 2 | 2 | 2 |
| 6 | 8112900002 | Power Cord | 1 | 1 | 1 | 1 |
| 7 | 8510100002 | User Manual | 1 | 1 | 1 | 1 |
| 8 | 8510120001 | Checking List | 1 | 1 | 1 | 1 |
| 9 | 8310100008 | Ground Cable | 1 | 1 | 1 | 1 |
| 10 | 8189007074 | Adult NIBP cuff with connector, Sac-type (for 25-35cm) | 0 | 0 | 1 | 1 |
| 11 | 8189007010 | NIBP Extension Hose | 0 | 0 | 1 | 1 |
| 12 | 8310400014 | Reusable Adult SpO2 Probe | 0 | 0 | 1 | 1 |
Options:
| No. | Parts No. | Description |
| NST Report | ||
| 1 | N/A | Fisher NST Report (Fetal Health Report) |
| Transducer | ||
| 2 | 8310400004 | FHR Transducer |
| N/A | Waterproof FHR Transducer | |
| 3 | 8310400003 | TOCO Transducer |
| N/A | Waterproof TOCO Transducer | |
| 4 | 8310400012 | Event Marker |
| Recording Paper | ||
| 5 | 8111810003 | Recording Paper (112mmx100mmx150p, European Standard, per piece) |
| Recording Paper (112mmx100mmx150p, US Standard, per piece) | ||
| NIBP | ||
| 6 | 8189007010 | NIBP Extension Hose |
| 7 | 8189007074 | Adult NIBP cuff with connector, Sac-type (for 25-35cm) [for other special size, please contact the salesman.] |
| SPO2 | ||
| 8 | 8310400014 | Reusable Adult SPO2 sensor with plug |
| Others | ||
| 9 | 8112600002 | Touch Screen |
| 10 | N/A | Computer Keyboard |
| 8490000047 | Mouse | |
| 11 | USB port for patient data/NST report backup | |
| 12 | 8600000001 | Central Monitoring Station (software + dongle) |
| 13 | 8212010009 | Rechargeable Lithium-ion Battery |
| 14 | 8490000012 | Fetal Acoustic Stimulator |
| 15 | 8189007066 | Belt (per piece) |
| 16 | 8190100001 | Ultrasound Gel (250g) |
| 17 | 8212900052 | Wall Mounting Rack with Basket |
| 18 | 8510110002 | Service Manual |
| 19 | 8490000001 | Trolley with basket and wheels |
| 20 | 8700010103 | Net Cable for Central Station (5M) |
| 21 | 8112900002 | Power Cord (3 pin) |
| 22 | 8310100008 | Ground Wire |
| 23 | N/A | USB Input Function (support USB Mouse/Keypad input and control) |
| N/A | USB Output Function (patient data backup by USB and NST report) | |
| 24 | 8490000031 | Wireless AP (transmitter & Receiver) (for wireless central monitor system) |
| 25 | 8490000030 | Wireless AP (transmitter) (for wireless central monitor system) |
| 26 | 9100010003 | Switch Board |
| * Contents are subject to change without prior notice | ||